We had the pleasure to interview Prof. E. Peterman and Guus Haasnoot from the Physics of Living Systems, Department of Physics and Astronomy, Faculty of Science, Vrije Universiteit in Amsterdam, The Netherlands, and learn more about their research on living C. elegans. Their research focuses on chemosensory cilia—specialized neuron extensions that allow these microscopic worms to sense their environment.
What is your research about?
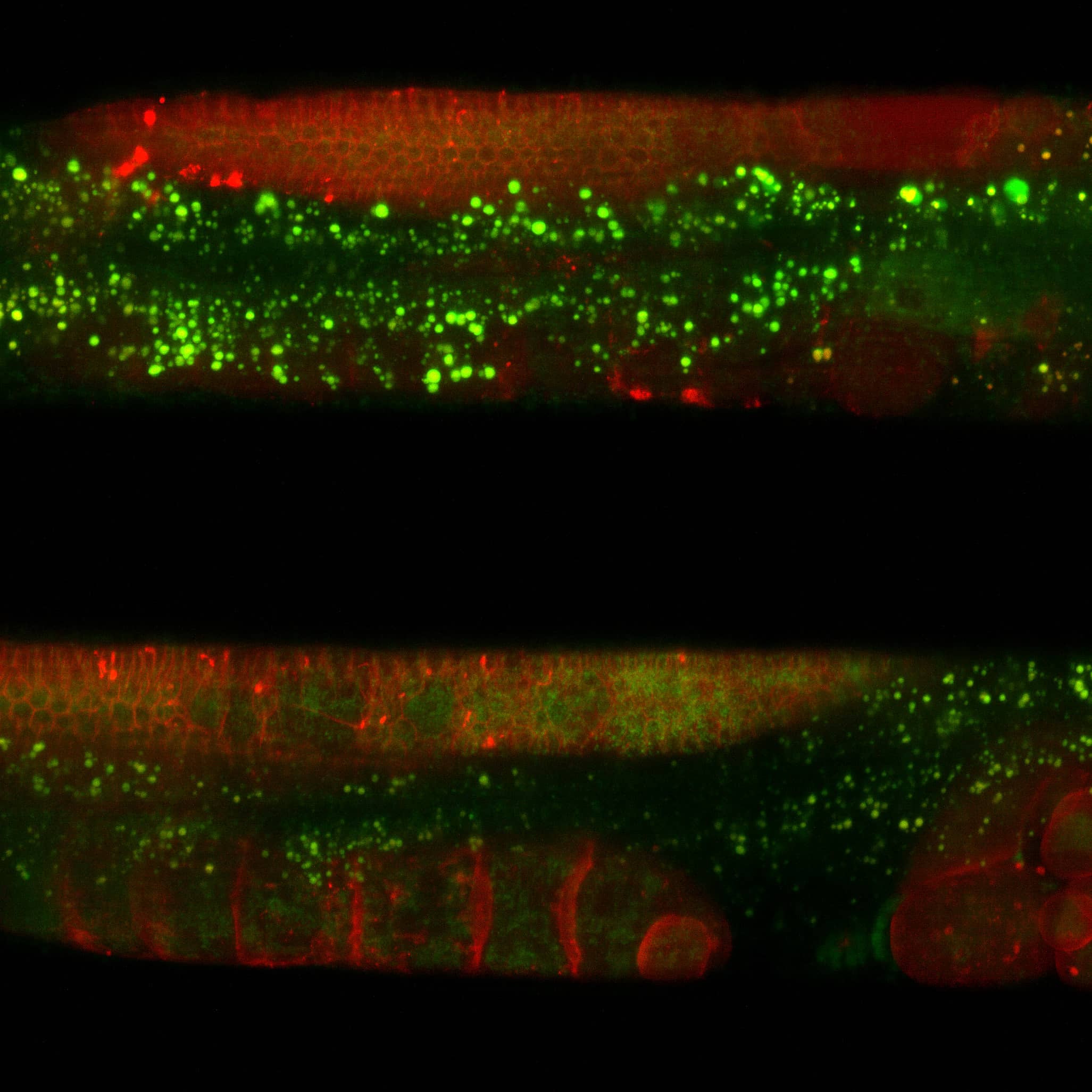
We study C. elegans, and in particular, we study cilia in C. elegans. Cilia are extensions of some of the neuron cells in C. elegans. In fact, they act as a sort of antenna, connecting the animal with the outside world and, in particular, measuring, sensing or maybe tasting is the better word, chemical compounds in the environment. We try to understand how this sensory mechanism works in living worms. This works with these cilia, these special organelles, and we really need fancy microscopes to look at them and to see how they work.
What is your research objective?
Overall, the goal of my research is to understand how life processes work and what their physical origins are. And in the cilia in these worms, there are several of these processes that we are really excited about. We try to understand how certain proteins end up at a certain location, as a combination of active transport driven by motors, intraflagellar transport, but also, diffusion, random motion. We try to understand how that works, but also, we try to understand how this actual sensing works, how the interaction of a single molecule with a single receptor, how that translates to an avalanche of effects, neuronal activity and then ultimately to changes in behavior. And that’s what’s so fascinating.
Why do you study living C. elegans?
I had been studying them (motor proteins) for a long time in in vitro conditions. We have been working with isolated proteins, working together under the microscope and looking at what’s happening, and that’s great, it’s fascinating, you can learn a lot. But biology in the cell is so much more complex. That’s why we switched to an in vivo system. I was introduced to that system by Jon(athan) Scholey (UC Davis) (a collaborator), and that really opened my eyes. It’s so much nicer to really look into this in vivo system and to really try to understand life in its full complexity, and C. elegans is really super unique in the sense that you can really go from the single molecule level to really the behaviour of the whole animal. I don’t think that’s possible with any other model system.
What is the role of imaging in your research?
Imaging, and to be more precise, fluorescence microscopy, is the cornerstone of our research. We wouldn’t be able to see anything without a microscope. Things are too small, and we need fluorescence for the specificity. We are interested in single-molecule behavior and cells contain thousands, millions of proteins. But we really need to make sure that only one of them is labeled and visible. We need sensitivity and specificity, and only fluorescence microscopy can bring that to us.
What are the essential characteristics of live cell imaging to you?
We study living systems, and for that, it’s really important, first of all, to be sensitive. We need very high sensitivity, very high specificity, and suppressed background. Another crucial thing is that imaging needs to be fast. Maybe the most important one is that imaging needs to be gentle. There are several reasons for that: Photobleaching is where the fluorescence signal decreases over time. That makes your images fade; That’s not good. More importantly, too much light can be toxic to the animal. And then we’re really changing all sorts of processes in the animal and that’s not what we want.
How did the NL5+ help you accelerate your research?
The NL5 has enabled us to measure in different parts of the animal what we so far could not measure properly. The animal is relatively thick in certain places, and that makes imaging difficult. The NL5 really helps suppress the background. Furthermore, what we really noticed is the ease of use and the relative simplicity as a user of this instrument that really allows you to play a lot with the settings and find the things that work. And what really stands out is that it’s very gentle to the specimen. We can really measure very long times and get very long sequences of images, and that really benefits our research.
Guus Haasnoot – What is your research about?
In our lab, we look at these chemosensory cilia, and we look at how they function and, specifically, their transport. In my research, I look more into how they actually sense. To do this, we put the worm inside of a very small microfluidic chip, and then we can, very precisely, expose the worms to different kinds of chemicals, and then we can study, with our NL5 system, how the cilia and the whole nervous system, reacts to these types of stimuli.
Guus Haasnoot – What is important to your research regarding imaging?
What is important for my research is that, especially when I look at the cilia, we need optical sectioning. In the tail of the worm, there are only one or two cilia, but in the head of the worm, there are many cilia that are combined. And, of course, this is in 3D. So we need this optical sectioning to really get a better contrast, on the processes that we see. We are looking at processes of tiny motor proteins, with very low signal, and that’s why we also need the high sensitivity of the system.
We would like to thank Prof. E. Peterman and Guus Haasnoot for this insightful interview.